Lab Report On Buffer Preparation . learn how to make equilibration, wash and elution buffers for protein purification experiments. Designing and preparing a buffer. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid,. the objective of the experiment conducted was to give students the understanding the derivation of the henderson. prepare a buffer from an acid and its conjugate base components. learn the properties and factors of buffer solutions by preparing and measuring different buffer solutions from acetic acid. Titrate buffers with a strong acid and a strong base. Find out how to test for buffering. prepare 50 ml from phosphate buffer with concentration 0.25m and ph=7.4, if you know that (pka=7.2). a buffer is a solution that resists changes to ph when a strong acid or base is added to the solution.
from www.chegg.com
The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid,. Designing and preparing a buffer. Titrate buffers with a strong acid and a strong base. a buffer is a solution that resists changes to ph when a strong acid or base is added to the solution. prepare 50 ml from phosphate buffer with concentration 0.25m and ph=7.4, if you know that (pka=7.2). the objective of the experiment conducted was to give students the understanding the derivation of the henderson. learn the properties and factors of buffer solutions by preparing and measuring different buffer solutions from acetic acid. Find out how to test for buffering. learn how to make equilibration, wash and elution buffers for protein purification experiments. prepare a buffer from an acid and its conjugate base components.
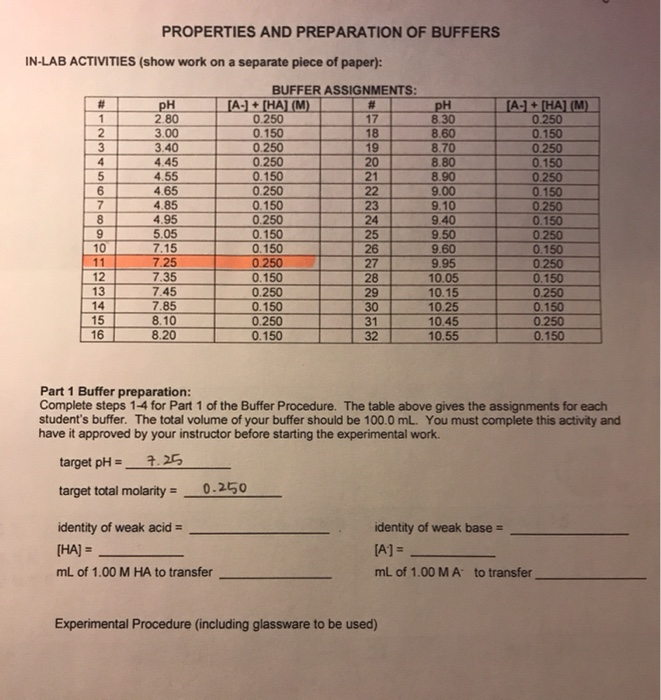
Solved PROPERTIES AND PREPARATION OF BUFFERS INLAB
Lab Report On Buffer Preparation the objective of the experiment conducted was to give students the understanding the derivation of the henderson. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid,. prepare a buffer from an acid and its conjugate base components. Titrate buffers with a strong acid and a strong base. learn the properties and factors of buffer solutions by preparing and measuring different buffer solutions from acetic acid. Find out how to test for buffering. prepare 50 ml from phosphate buffer with concentration 0.25m and ph=7.4, if you know that (pka=7.2). the objective of the experiment conducted was to give students the understanding the derivation of the henderson. a buffer is a solution that resists changes to ph when a strong acid or base is added to the solution. learn how to make equilibration, wash and elution buffers for protein purification experiments. Designing and preparing a buffer.
From www.studocu.com
BCH 214 Preperation OF Acetate Buffers TITTLE PREPERATION OF ACETATE Lab Report On Buffer Preparation Find out how to test for buffering. Titrate buffers with a strong acid and a strong base. learn the properties and factors of buffer solutions by preparing and measuring different buffer solutions from acetic acid. Designing and preparing a buffer. the objective of the experiment conducted was to give students the understanding the derivation of the henderson. The. Lab Report On Buffer Preparation.
From www.chegg.com
Solved Preparation of Buffer Solution Experiment I uploaded Lab Report On Buffer Preparation learn the properties and factors of buffer solutions by preparing and measuring different buffer solutions from acetic acid. learn how to make equilibration, wash and elution buffers for protein purification experiments. prepare 50 ml from phosphate buffer with concentration 0.25m and ph=7.4, if you know that (pka=7.2). a buffer is a solution that resists changes to. Lab Report On Buffer Preparation.
From www.studocu.com
Buffers Lab Report Complete the below pages and submit them to your Lab Report On Buffer Preparation The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid,. a buffer is a solution that resists changes to ph when a strong acid or base is added to the solution. learn the properties and factors of buffer solutions by preparing and measuring different buffer solutions from acetic acid. . Lab Report On Buffer Preparation.
From www.chegg.com
Buffer lab report ? Lab Report On Buffer Preparation The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid,. prepare a buffer from an acid and its conjugate base components. Find out how to test for buffering. Titrate buffers with a strong acid and a strong base. the objective of the experiment conducted was to give students the understanding. Lab Report On Buffer Preparation.
From www.labcompare.com
A Guide to Biological Buffer Preparation Lab Report On Buffer Preparation the objective of the experiment conducted was to give students the understanding the derivation of the henderson. Find out how to test for buffering. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid,. Designing and preparing a buffer. prepare 50 ml from phosphate buffer with concentration 0.25m and ph=7.4,. Lab Report On Buffer Preparation.
From dxonofogy.blob.core.windows.net
Buffer Solutions Laboratory at Danny Wetzler blog Lab Report On Buffer Preparation Designing and preparing a buffer. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid,. prepare a buffer from an acid and its conjugate base components. Titrate buffers with a strong acid and a strong base. the objective of the experiment conducted was to give students the understanding the derivation. Lab Report On Buffer Preparation.
From theuniverypapers.web.fc2.com
Order Paper Writing Help 24/7 buffers lab report 2017/09/29 Lab Report On Buffer Preparation Find out how to test for buffering. a buffer is a solution that resists changes to ph when a strong acid or base is added to the solution. prepare a buffer from an acid and its conjugate base components. Titrate buffers with a strong acid and a strong base. The dissociation of a weak acid in water yields. Lab Report On Buffer Preparation.
From www.slideserve.com
PPT Experiment 7 Preparation and Properties of Buffers PowerPoint Lab Report On Buffer Preparation prepare a buffer from an acid and its conjugate base components. the objective of the experiment conducted was to give students the understanding the derivation of the henderson. learn the properties and factors of buffer solutions by preparing and measuring different buffer solutions from acetic acid. The dissociation of a weak acid in water yields h+ ion. Lab Report On Buffer Preparation.
From www.chegg.com
Preparation of Buffer Solutions PRELAB QUESTIONS 1. Lab Report On Buffer Preparation Titrate buffers with a strong acid and a strong base. learn how to make equilibration, wash and elution buffers for protein purification experiments. learn the properties and factors of buffer solutions by preparing and measuring different buffer solutions from acetic acid. the objective of the experiment conducted was to give students the understanding the derivation of the. Lab Report On Buffer Preparation.
From www.studocu.com
Lab 11 Buffers LAB Lab 11 Examination of Buffer Solutions Lab Report On Buffer Preparation prepare a buffer from an acid and its conjugate base components. Designing and preparing a buffer. a buffer is a solution that resists changes to ph when a strong acid or base is added to the solution. prepare 50 ml from phosphate buffer with concentration 0.25m and ph=7.4, if you know that (pka=7.2). The dissociation of a. Lab Report On Buffer Preparation.
From www.studocu.com
Bufferpreparation Buffer Solution Buffer Preparation (Shi Lab) 1 M Lab Report On Buffer Preparation The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid,. learn how to make equilibration, wash and elution buffers for protein purification experiments. Titrate buffers with a strong acid and a strong base. the objective of the experiment conducted was to give students the understanding the derivation of the henderson.. Lab Report On Buffer Preparation.
From studylib.net
Lab 19 Buffers Lab Report On Buffer Preparation Designing and preparing a buffer. prepare a buffer from an acid and its conjugate base components. Titrate buffers with a strong acid and a strong base. learn the properties and factors of buffer solutions by preparing and measuring different buffer solutions from acetic acid. the objective of the experiment conducted was to give students the understanding the. Lab Report On Buffer Preparation.
From www.chegg.com
Buffer lab report ? Lab Report On Buffer Preparation Titrate buffers with a strong acid and a strong base. prepare a buffer from an acid and its conjugate base components. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid,. learn how to make equilibration, wash and elution buffers for protein purification experiments. learn the properties and factors. Lab Report On Buffer Preparation.
From www.academia.edu
(PDF) Experimental Report 13 " pH Buffer Solutions " Mariana Lab Report On Buffer Preparation a buffer is a solution that resists changes to ph when a strong acid or base is added to the solution. prepare a buffer from an acid and its conjugate base components. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid,. prepare 50 ml from phosphate buffer with. Lab Report On Buffer Preparation.
From www.slideserve.com
PPT Buffer This PowerPoint Presentation ID2841454 Lab Report On Buffer Preparation prepare a buffer from an acid and its conjugate base components. the objective of the experiment conducted was to give students the understanding the derivation of the henderson. Designing and preparing a buffer. learn the properties and factors of buffer solutions by preparing and measuring different buffer solutions from acetic acid. Find out how to test for. Lab Report On Buffer Preparation.
From www.slideshare.net
Preparation of p h buffer solutions Lab Report On Buffer Preparation prepare a buffer from an acid and its conjugate base components. learn the properties and factors of buffer solutions by preparing and measuring different buffer solutions from acetic acid. the objective of the experiment conducted was to give students the understanding the derivation of the henderson. a buffer is a solution that resists changes to ph. Lab Report On Buffer Preparation.
From www.studocu.com
Buffer lab report Lab 5 Efficacy of Buffers A Lab to Determine the Lab Report On Buffer Preparation learn the properties and factors of buffer solutions by preparing and measuring different buffer solutions from acetic acid. the objective of the experiment conducted was to give students the understanding the derivation of the henderson. prepare 50 ml from phosphate buffer with concentration 0.25m and ph=7.4, if you know that (pka=7.2). learn how to make equilibration,. Lab Report On Buffer Preparation.
From www.studocu.com
Lab 3 report Laboratory N° 3 Biological Buffers IIPreparation of a Lab Report On Buffer Preparation prepare a buffer from an acid and its conjugate base components. learn how to make equilibration, wash and elution buffers for protein purification experiments. the objective of the experiment conducted was to give students the understanding the derivation of the henderson. a buffer is a solution that resists changes to ph when a strong acid or. Lab Report On Buffer Preparation.